Long-Term Records of CO2 Pressure Fluctuations by STEPHAN KEMPE, Hamburg*) Contents
*) Address of the author: Dr. STEPHAN KEMPE Geologisch-Paläontologisches Institut und Museum der Universität Hamburg, Bundesstraße 55, 2000 Hamburg 13, Bundesrepublik Deutschland 3. Groundwater Case Study: The Carbonate/Gypsum Karst of The dominating role of dissolved CO2 in weathering and chemical erosion of continents is well established (e. g. GARRELS & MACKENZIE, 1971). However, textbooks on groundwater and karstology are principally concerned with the thermodynamics of CO2 reactions but say little on specific geological situations, namely case studies (MATTHESS, 1973; RICHTER & LILLICH, 1975; PICKNETT et al., 1976; BÖGLI, 1978). Prior to the use of portable pH-meters and fully computerized thermodynamic electrolyte models, CO2 concentrations were often approximated by the TILLMANNS equation (TILLMANNS & HEUBLEIN, 1912):
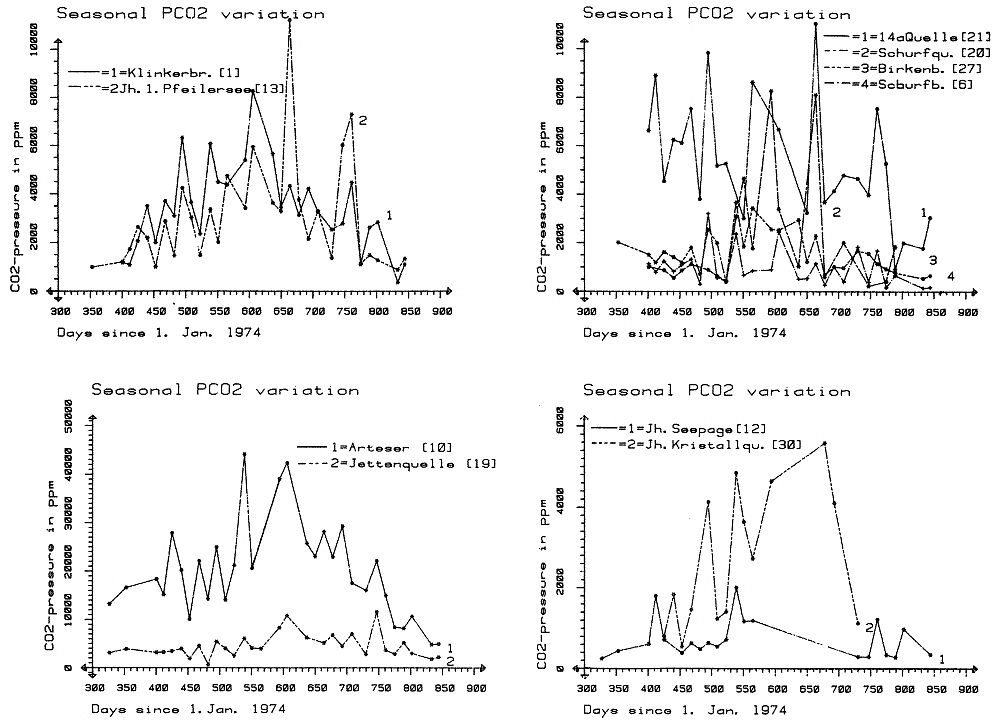
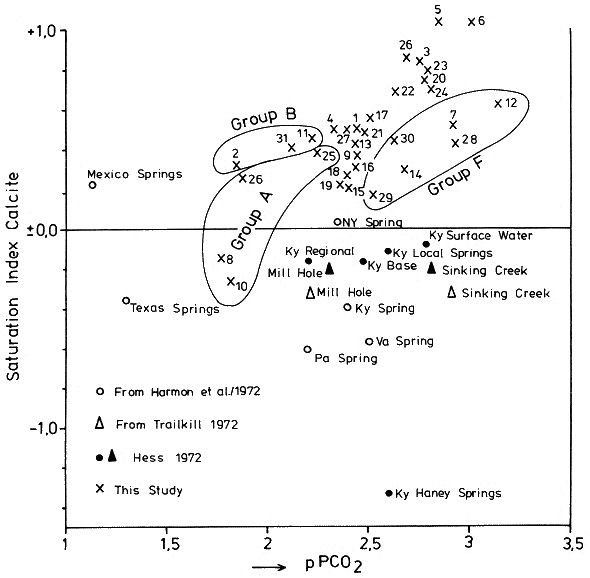
which is a measure of the amount of CO2 needed to hold a given amount of calcium carbonate in solution. The Tillmanns constant "K" is temperature dependent. To estimate the "aggressive" free CO2, that is the CO2 exceeding the amount calculated by the Tillmanns equation, a series of techniques were developed using pH-measurements or direct determination of the total free CO2 (these techniques are discussed in detail by MATTHESS, 1973). Monitoring of small PCO2 and mineral saturation variations with time over a range of groundwater samples derived from various sources has become feasible relatively recently through the development of precise computerized thermodynamic calculations and sample evaluation. As the question of the degree of saturation with respect to carbonate minerals is especially interesting for the understanding of the geochemistry of karsts, accurate PCO2 calculations are based largely on field studies from these areas (e. g. PARIZEK et al., 1971; HARMON et al., 1972; TRAILKILL, 1972; WIGLEY et al., 1973; HESS, 1974; FLEYFEL, 1979). Saturation of carbonate minerals was related to the type of aquifer (PARIZEK et al., 1971), for instance diffuse flow through limestone results in higher carbonate mineral saturation and mostly higher PCO2 when compared to conduit flow. In addition, mean annual temperature influences the mean PCO2 (HARMON et al., 1972). To illustrate the effect of temperature on PCO2 we will briefly examine the region between Southern Mexico and the Northern United States where a groundwater temperature gradient of about 20° C exists. Due to higher respiration activity in the much warmer tropical and subtropical soils , the PCO2 of groundwaters differs between the two areas by a factor of 10. In Mexico PCO2 values of 30 000 ppm were measured compared to a mean of 3000 ppm in the Northern U.S. karst regions (HARMON et al., 1972). PARIZEK et al. (1971) and HESS (1974) also report substantial PCO2 variations in karstic groundwaters, due to seasonal effects. The first comparative study of PCO2 from a variety of geologic settings in a Central European karst region was conducted by the author and co-workers in May 1973 in the Zechstein area South of Osterode/Harz, West Germany (BRANDT et al., 1976) (Fig. 2). A total of 41 water samples from gypsum-, limestone-, and nonkarstic waterbodies were analysed and the PCO2 calculated. The results showed a large scatter of values between 300 and 20 000 ppm. Due to monoseasonal sampling it could not be decided if this scatter was due to genuine environmental differences in the sources sampled or due to different phase shifts in the seasonal PCO2 curve for the individual sample locations. To settle these questions and to obtain the absolute level of PCO2 and the magnitude of seasonal fluctuations for individual water sources a one and a half year hydrochemical monitoring was conducted in the area between November 1974 and April 1976. The results of this field experiment are presented below.
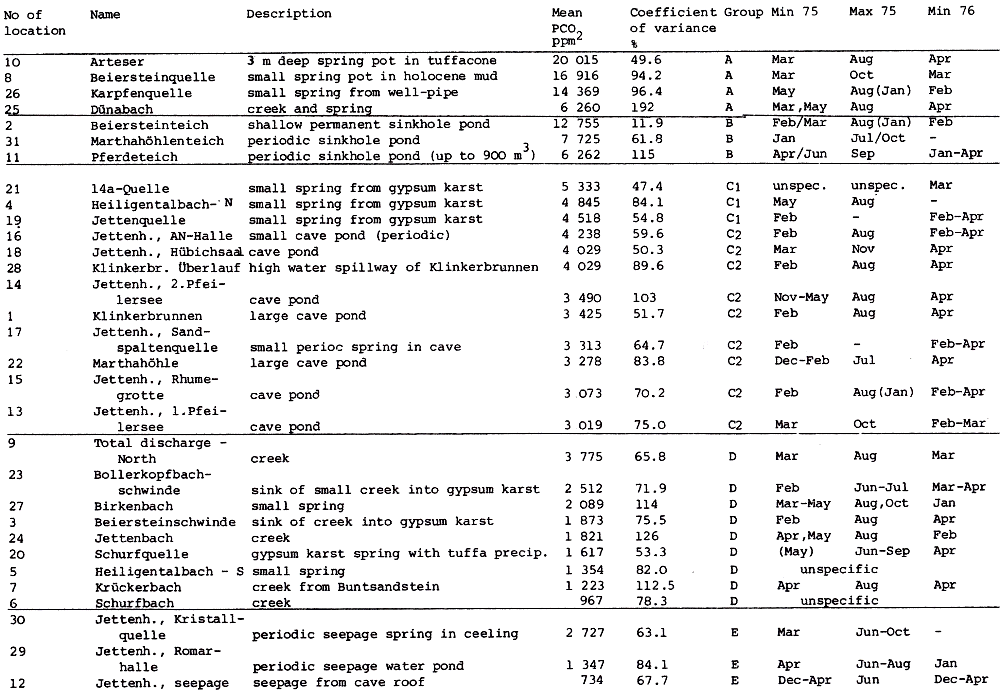
Table 4
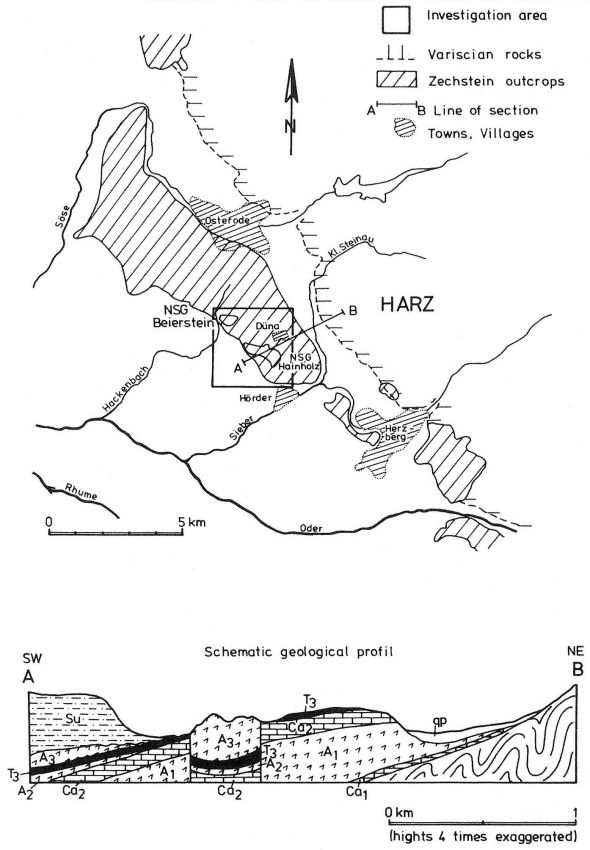
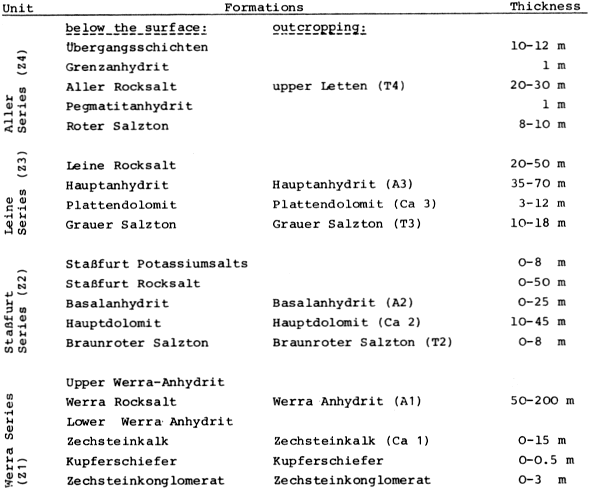
The upper Permian (Zechstein) salinar series of the South Harz/Germany consists of four cyclic stratgraphic units (Table 4) (HERRMANN, 1957). South of Osterode (Fig. 2), the Zechstein forms a two to three kilometer wide NNW-SSE extending outcrop. The former halite has been removed by subrosional processes. Only the anhydrite (changed partly to gypsum), limestone, dolomite, and clay formations are exposed at the surface. Uplift of the Variscian folded Harz Mountains has slightly tilted the Zechstein toward the SE. Locally, however, the earlier subrosion of the halite and the ongoing dissolution of the gypsum and carbonate series has caused much irregularity in the outcrop pattern. In addition, NW-SE and ENE-WSW striking faults disturb the stratigraphic order. Fig. 2 gives a schematic profile of the Zechstein outcrop. The A1, the Werra Anhydrite forms a prominent white escarpment which is capped by the Ca-2, the Staßfurt-, Haupt-, or Stinkdolomite which in places is covered by the up to 15 m thick T 3, the Grauen Salzton. These two formations form the Plateau of Düna. Toward the south, the intercalated A 2 or Basalanhydrite is gradually vanishing. Faults have lowered the originally up to 70 m thick A 2 or Hauptanhydrite to the level of the Düna Plateau, and by that protected this formation from becoming eroded. The Zechstein suite is buried by the steep escarpment of the lower Buntsandstein which also hides the thin beds of the Zechstein-4 or Aller-series (KEMPE et al., 1970. The Hauptanhydrite-Karst Hainholz and a smaller outcrop toward the NW - the Beierstein - form, together with the surrounding dolomite plateau and the clay and siltstone terrain of the Buntsandstein, a perfect hydrochemical test ground within a very small area. In addition, the karst has been declared a conservation area since 1967 minimizing the influence of human activity (KEMPE, 1979 c). The general hydrochemistry has been worked out in 1973 (Fig. 3). A morphologic water devide between the Hackenbach to the North and the Sieber to the South, straddles the area, but underground direction of karst drainage remains largely unknown. For details see BRAND et al. (1976). |
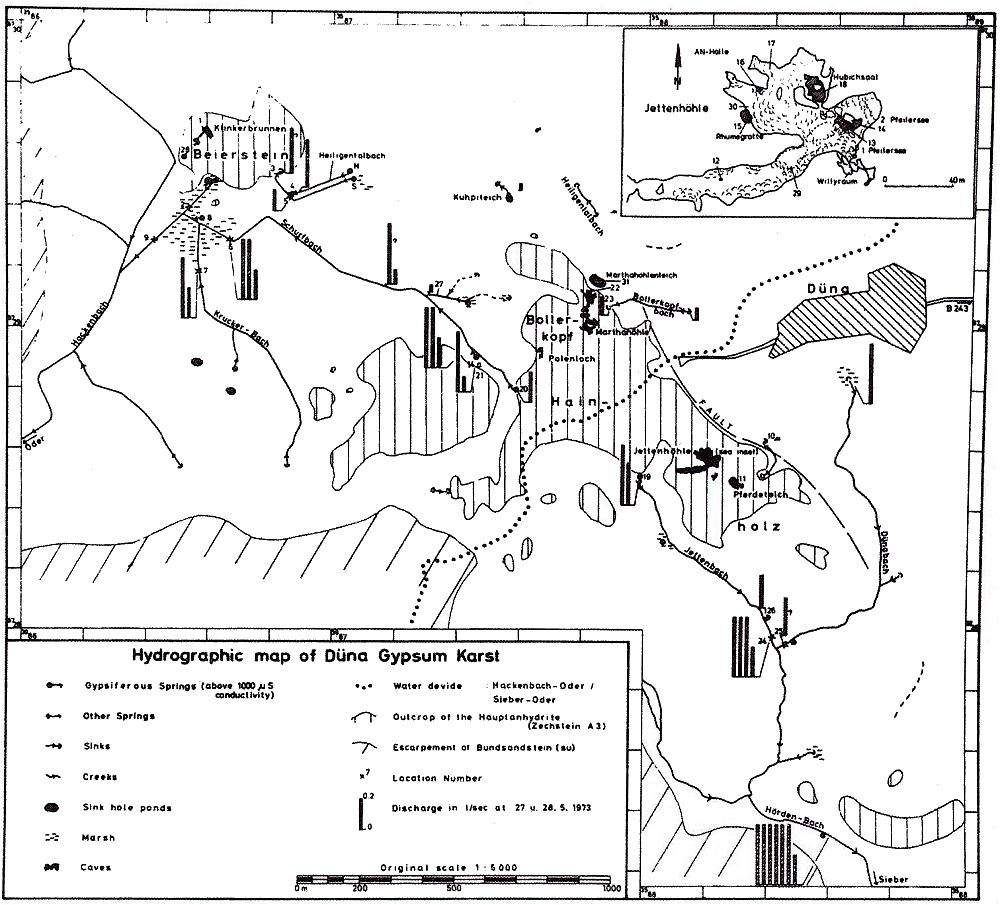
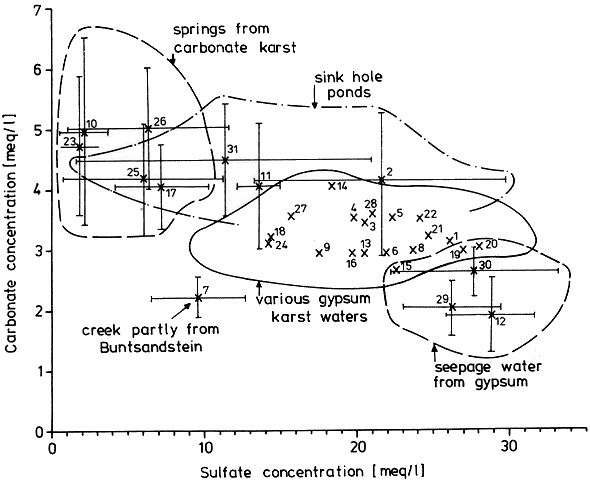
Fig. 3 Geologic-hydrographic map of the Hainholz and Beierstein gypsum karsts and the surrounding area with locations of sampling stations,
November 1974 - April 1976.
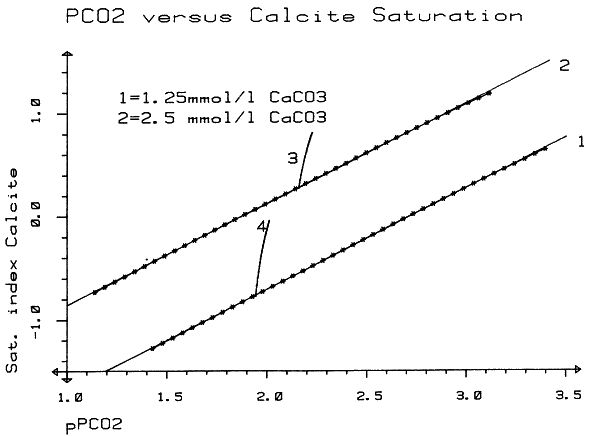
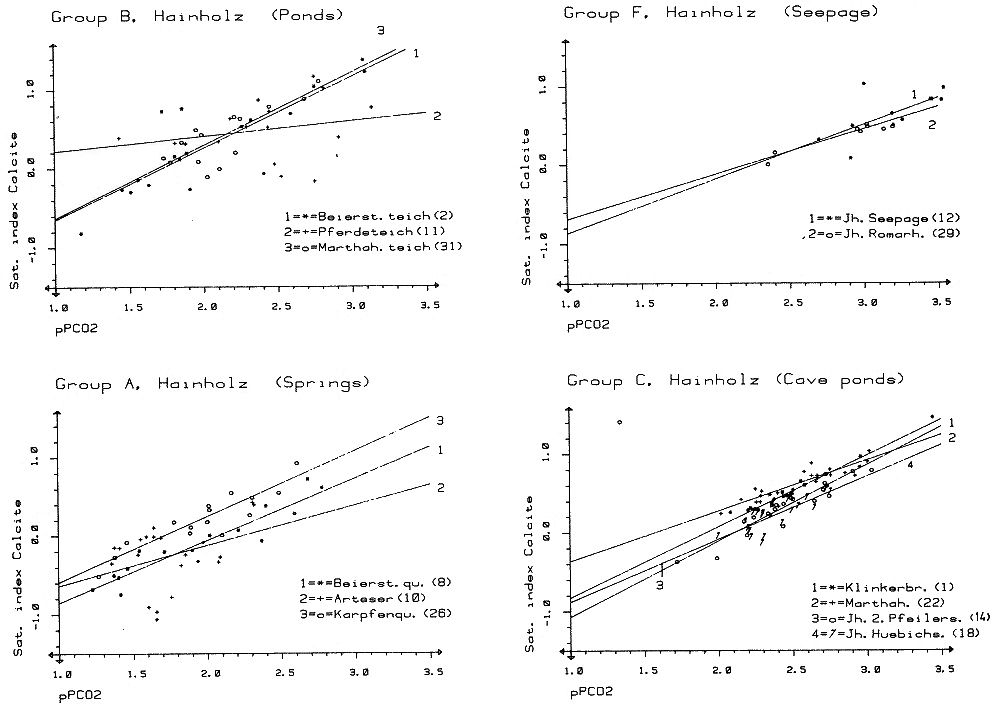
Within the gypsum beds some thirty caves are known, the largest being the Jettenhöhle (KEMPE et al., 1972). These caves develop by solution in the standing groundwater and enlarge their volume by ceiling breakdown into the groundwater outcrops (BIESE, 1931; KEMPE, 1970; KEMPE & SEEGER, 1972; KEMPE et al., 1976). These caves give direct access to the karst groundwater. Thus, not only geologically and hydrochemically titally different aquifers can be sampled from the Hainholz area but water can be collected from a varity of hydrological settings: (i) from surface creeks, (ii) from karst and subsurface runoff springs, (iii) from sinkhole ponds, (iv) from groundwater outcrops within caves and (v) from seepage water of the cave roof. A total of 31 sample locations were selected within the Hainholz and the sourrounding terrain (Fig. 3). Starting in fall 1974, these locations were sampled on average every fourteen days. Appendix 1 gives dates and number of samples taken and analysed. A total of 933 samples were taken during this exercise. Temperature and pH of the water were directly measured. For pH determinations the battery powered KNICK-Portamess with analog display and temperature compensation was used in conjunction with a plastic protected INGOLD-450 combination reference pH electrode. The electrode was standardized against MERCK buffers pH 4, 7, and 9 several times during the sampling walk. The accuraty of the readings are within ± 0.05 pH. The samples were bottled by letting the water slowly run into 100 ml polyethelene containers and closing the bottles under water without trapping any air in the bottle. The alkalinity was titrated immediately after returning to the field station. From a glass burette 0.02 n HCL was added to 20 ml sample plus 3 drops methylorange (0.1 % solution). The titration end point was a barely persisting onionred (MERCK, 1974). The burette could be read to 0.02 ml. The precision of the analyses should therfore be ± 0.02 meq/l. Following the settling of sediment matter, the total hardness was determined in 10 ml of water with a 0.1 m EDTA solution. One half to one third indicator tablet and 3 drops of 25 % NH3 were added as indicator. The titration end point was a persistant green (MERCK, 1974). The analytical precision is about ±0.01 meq/l. Some of the Ca- and Mg-organic complexes take time to disintegrade under the influence of EDTA which introduces a slight error in waters containing high Ca and Mg concentations (KEMPE, 1975 b). Samples were stored at 4° C to be later analysed for dissolved Ca and Mg by atomic absorption techniques using lanthanium chloride as a buffer. For Ca, Mg, Na, and K is ±1.5 % (1 standard deviation). Sodium and potassium concentrations almost follow each other in all types of water; concentrations are low. Only the Dünabach (sample location 25) has a K value of 1.4 mg/l most likely due the runoff of cattle sewage from the village (not included in the mean value). The strontium concentration has a mean (61 measurements from two samples series) of 1.13 mg/l with the standard deviation of 0.65 or 57.3 %. The samples from the Heiligentalbachtal (Location 3, 4, 5) and the Klinkerbrunnen (1.28) have values of above 2 mg/l which suggests that the water of the Beierstein creek sink (Location 3) reappears in the Klinkerbrunnen. In calculating the PCO2 and mineral saturation indices with the computer program fixed concentrations for sodium (4.2 mg/l), potassium (0.08 mg/l), and Cl (15 mg/l) were used. The program further assumes the SO4-concentration to be equal to the difference between the meq/l concentrations of (Ca + Mg + Na + K) and (HCO3 + Cl). In those cases where no Ca and Mg determination was available, the program takes the total hardness value for Ca and calculates the PCO2 without any iteration step. These CO2 values are therefore less accurate than those calculated from complete analyses. Analytical data, PCO2, and saturation, indices for calcite, dolomite, and gypsum are listed in Tables Appendix 2-32 together with the total column statistics. The number of days starts with the first of January 1974 and the samples are identical with the numbers in Table Appendix 1. According to Average PCO2 In Table 5 all sample locations are ranked according to their respective overall mean PCO2 values. These values are closed to the mean annual values as the record of the winter 1974/75 is rather incomplete and thus prevents the means to be biased too much toward winter pressures. |
Table 5
Sampling locations of the hydrochemical monitoring Nov 1974 - Apr 1976,
Hainholz/South Harz grouped according to average CO2 pressure